Gumboro disease (IBDV) is one of the most important immunosuppressive diseases of chickens that causes high economic losses to the poultry industry worldwide. In recent years, several publications have described the great impact that the subclinical form of Gumboro disease has had on large South American farms where the circulation of the virus variant has been detected1. In Brazil, Gumboro disease was first detected in the 1970s2 and although the disease was considered well-controlled after the widespread use of commercial vaccines, novel variant IBDV strains have now emerged, posing new threats to the poultry industry.
Infectious bursal disease virus is classified into three main groups according to antigenic and virulence properties: classical virulent (cvIBDV), very virulent (vvIBDV) and antigenic variants (avIBDV)3. This last group has been recently classified into genogroups G2, G4, G5, G6, and G7 and it is distributed in specific regions around the world.
Brazilian antigenic variants (avIBDV) have been detected in flocks with mild immunosuppression and low mortality related to secondary infections, but without presenting classical signs of Gumboro disease. Of several samples analyzed in a study in Brazil4, 35.42% were classified as avIBDV which were previously classified as GM15 and GM165.
Objective
The objective of this study was to evaluate the efficacy of new generation immune complex (GUMBOHATCH®) in Brazilian farms where variant Gumboro virus was circulating.
GUMBOHATCH® is a new immune complex vaccine against Gumboro disease that has been developed by HIPRA. This vaccine has introduced a different formulation (IgY of egg origin) and control parameters (detection of free IgY and neutralisation control) to guarantee complete coating of the IBDV vaccine virus at the time of inoculation.
The result of all these new improvements is a new-generation immune complex vaccine that allows maximum potency of the vaccine to be maintained and consistent results to be obtained in the field, whilst at the same time avoiding the risk of immunosuppression.
Methodology
Number of animals: 5,390,333 chicks
Vaccination: GUMBOHATCH®
Administration route: in-ovo at 18 days of incubation
Vaccination was performed in two cycles in accordance with the instructions in the package leaflet. All chicks were located in the north-east farms of Brazil after hatching:
- Cycle 1 in 80 commercial broiler farms
- Cycle 2 in 81 commercial broiler farms
All farms had been previously diagnosed with an avIBDV circulation, despite the fact that a vaccination programme based on the administration of standard formulated immune complex vaccine was in place.
Different safety and efficacy parameters were evaluated and monitored until the end of the rearing period (42 – 43 days of life).
- 15 birds/ farm at different times in each cycle
- Blood: antibody titres using CIVTEST® AVI IBD (HIPRA)
- Bursa of Fabricius: macro and microscopic lesions + PCR analysis + histopathology (29-33 days of age)
To evaluate the efficacy of GUMBOHATCH®, a comparison of the production results and the production cost differential from Cycle 1 (first contact of the farms with GUMBOHATCH®) vs Cycle 2 (stronger presence of the GUMBOHATCH® vaccine virus expected) was carried out.
Results
SAFETY
- No adverse reactions were observed on any of the farms regardless of the cycle.
- Expected signs of replication of the vaccine virus were observed in the bursas, but no lesions correlated with the field virus circulation were detected.
- Histopathology differences were observed in the bursas, with greater homogeneity of scores in Cycle 2 compared to Cycle 1 (data not shown).
EFFICACY
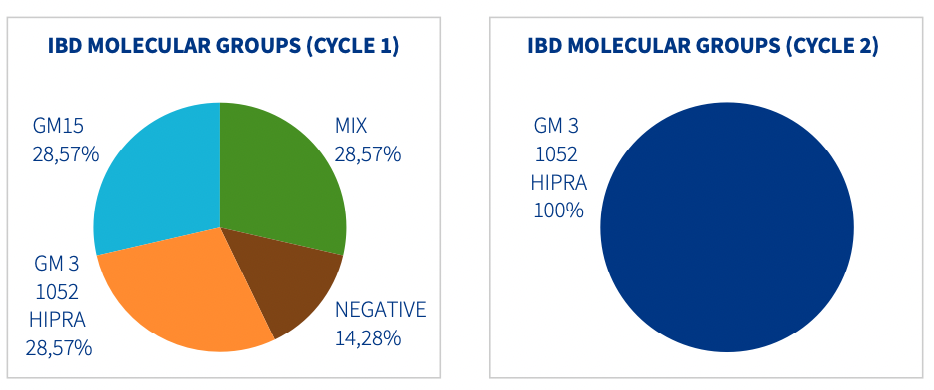
The serology results were as expected for GUMBOHATCH® in both vaccination cycles (Cycle 1 = mean titre 6141.14; Cycle 2 = mean titre 5593.47). However, major differences were seen between the PCR results for the two cycles (Figure 1).
- Cycle 1 = 28.75% of samples were positive for GUMBOHATCH®, 28.57% positive for virus variant (GM15), 28.57% were positive for both, GUMBOHATCH® + field virus and 14.28% of the samples were negative.
- Cycle 2 = 100% of the samples analyzed were positive for GUMBOHATCH®.
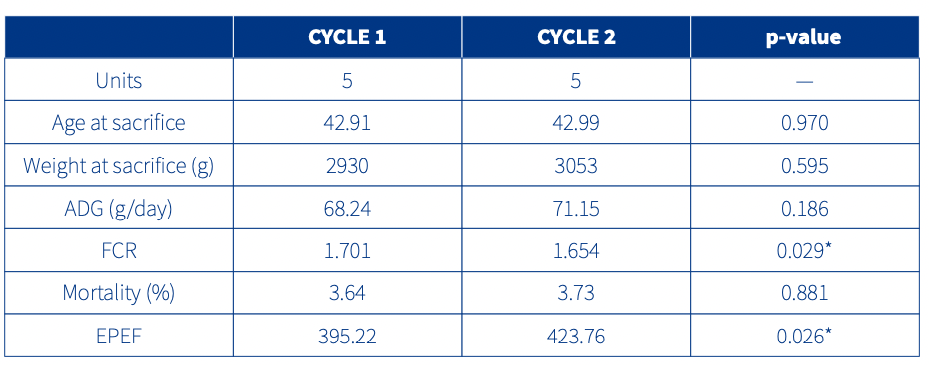
Moreover, a numerical improvement was seen in the majority of the productive parameters analysed in Cycle 2 compared to Cycle 1. The figures were significant in the case of the EPEF (European Production Efficiency Factor) and FCR (Feed Conversion Ratio) (Table 1).
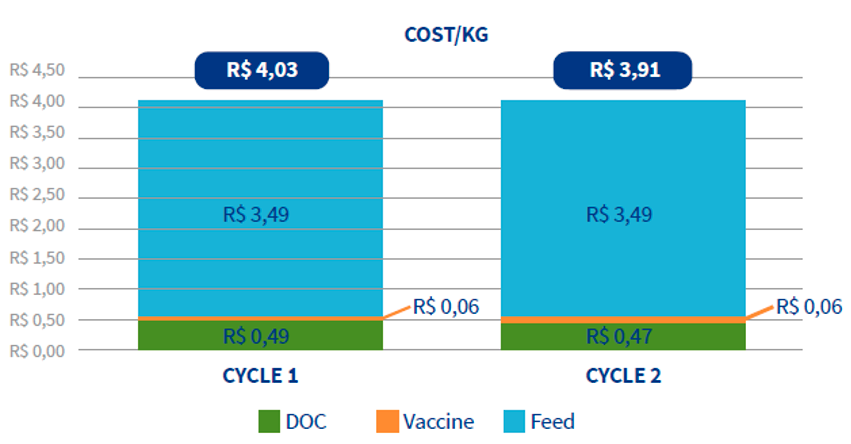
Additionally, in the second cycle the variable costs were reduced by R$ 0.12/kg (0.022 $/kg) live weight compared to the first cycle. Or in other words, in the second cycle with GUMBOHATCH®, the farms increased their profits by 22 R$ for each 1,000 kg of live weight produced.
Conclusions
The results obtained in this study allow the conclusion to be drawn that vaccination with GUMBOHATCH® is safe and confers adequate protection against circulation of the variant IBDV in chickens under field conditions in Brazil.
The differences observed in terms of efficacy between Cycle 1 and Cycle 2 appear to show that the vaccination programme based on the standard formulation immune complex vaccine was not providing adequate protection for the birds and field virus pressure had increased, with a negative impact on production results. The use of a new generation immune complex vaccine like GUMBOHATCH® allowed rapid colonization of the vaccine strain from one cycle to another, improving the immune status of the birds and production results.
Bibliography
1. Nakano et alt., 1972. Ocorrência da “Doença de Gumboro” no Brasil. Diagnóstico anátomo-patológico, Biológico (1972), 38:60-61.
2. Zachar et alt., 2016. Canadian Journal of Veterinary Research, Volume 80, Number 4, October 2016, pp. 255-261
3. Eterradossi and Saif, 2008. Infectious bursal disease. Diseases of Poultry. 12th ed. (2008), pp. 185–208.
4. Aline Padilha de Fraga et alt., Infection, Genetics and Evolution, Volume 73, September 2019, pp. 159-166
5. Ikuta et alt., 2001. Molecular characterization of Brazilian infectious bursal disease viruses. Avian Diseases (2001), pp. 297-306
