Immune complex Gumboro vaccines are formulated by mixing a well defined amount of an attenuated IBDV strain and a solution of specific antibodies against the same virus. But are all immune complex vaccines the same?GUMBOHATCH® is an immune complex vaccine with a new and unique formulation, in addition to introducing control parameters to ensure that all viral particles are completely coated with antibodies, that is, in the form of an immune complex. All these new improvements have resulted in a next generation immune complex vaccine, but, are there really benefits at the field level compared to other standard formulated immune complex vaccines?
Comparing GUMBOHATCH® with a standard formulated immune complex vaccine against Gumboro under field conditions in Brazil
A multicentre, positive-controlled and blind clinical trial was performed with the aim of evaluating the safety and efficacy of GUMBOHATCH®, when administered via the in-ovo route under field conditions, compared with a standard formulated immune complex vaccine in Brazil.
A total of 112,100 chicks were vaccinated in-ovo (18 days of incubation) with GUMBOHATCH® (n= 56,200) or with a standard formulated immune complex vaccine (n=55,900), following the manufacturer’s instructions.
After hatching, the chicks were distributed to 3 commercial broiler farms located in the Paraná state.
On each farm the two groups were housed in separate units under identical conditions and monitored up to the end of rearing (42 days of life). Several safety and efficacy parameters were evaluated during this period.
No adverse reactions to either of the two vaccines were observed and similar hatchability and body weight after hatching were also observed in both groups.
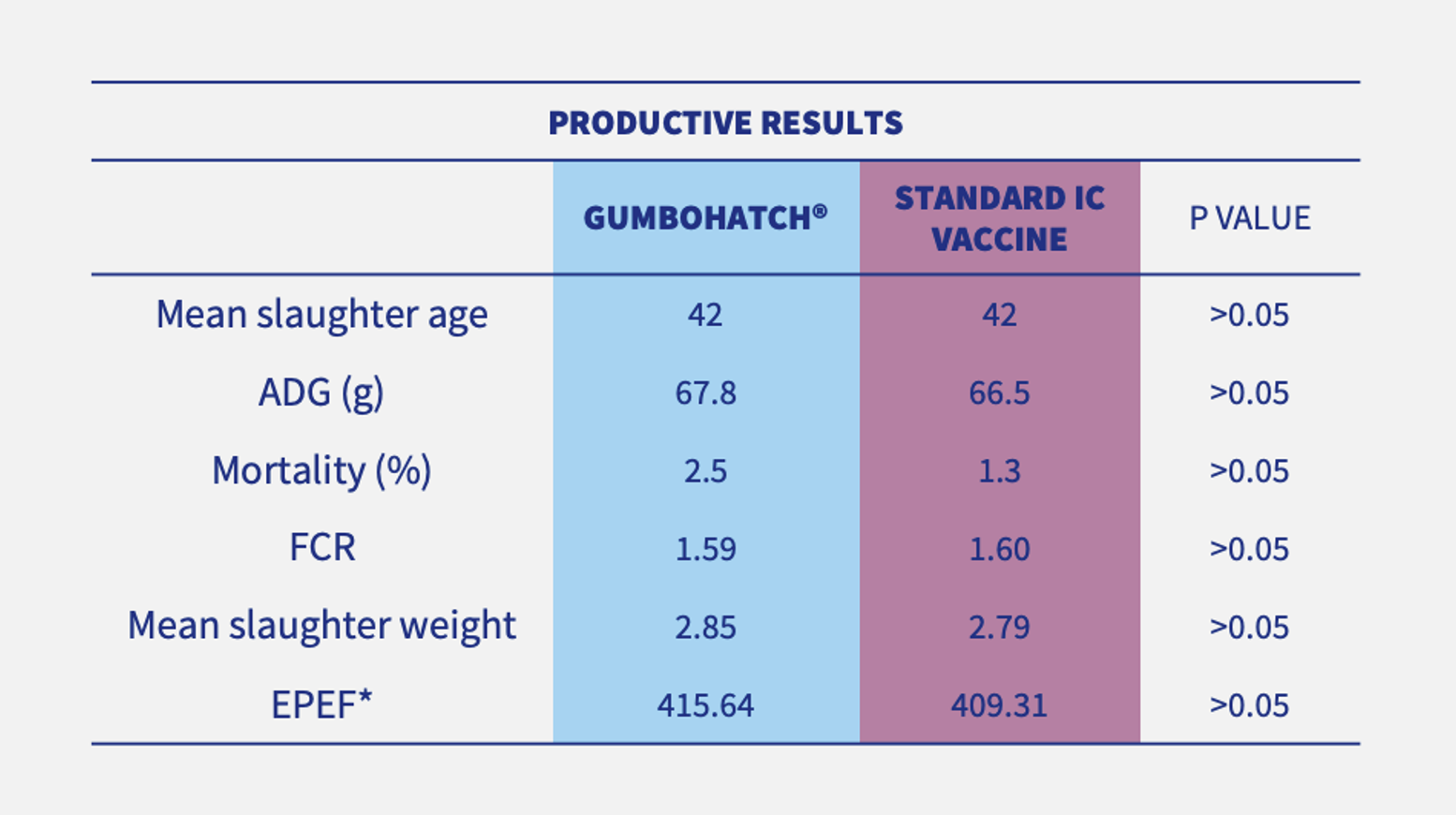
Although no Gumboro disease outbreak occurred on any of the farms, general productive parameters were slightly improved (numerically) in the case of houses vaccinated with GUMBOHATCH® (Table 1).
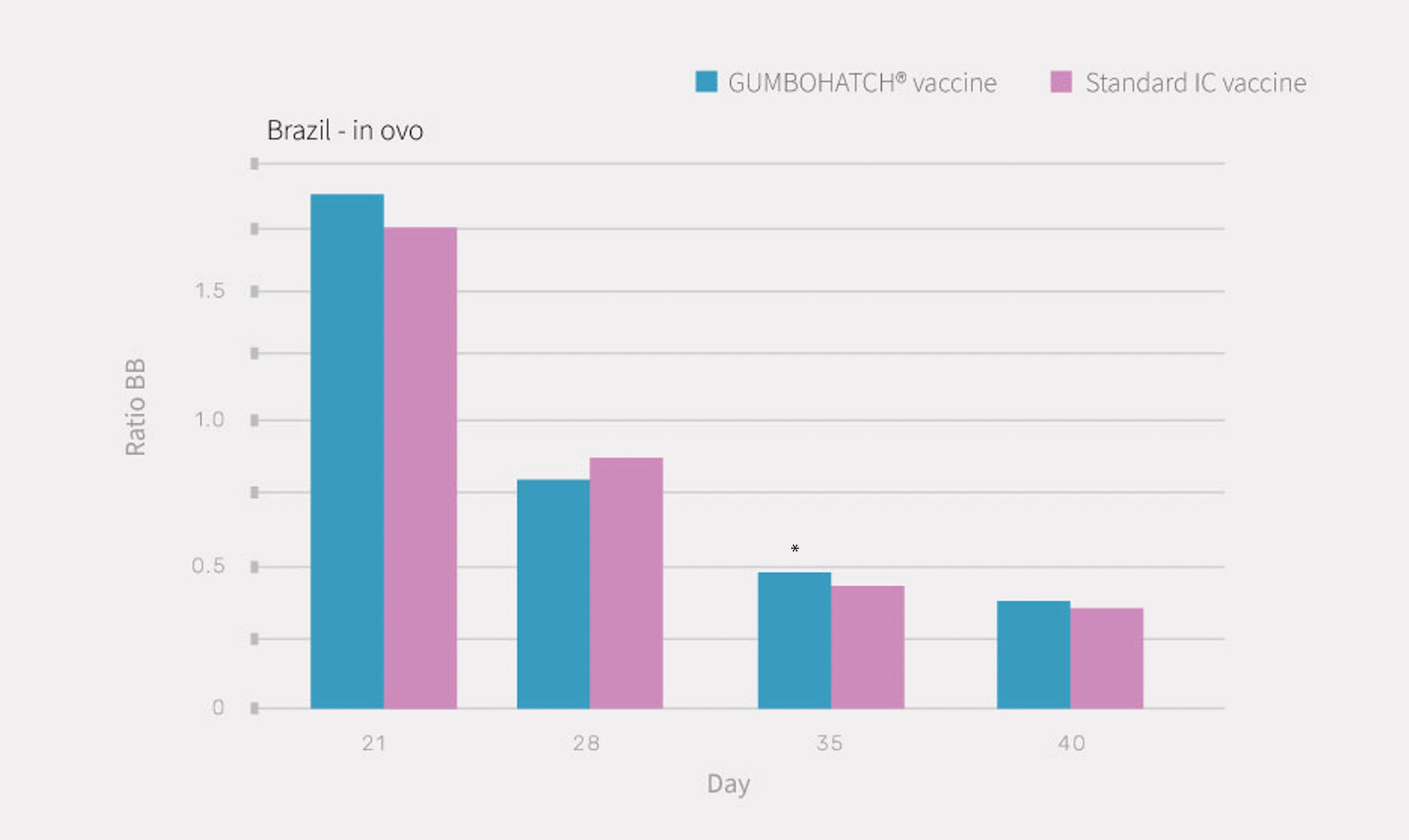
PCR results from bursal imprints evidenced replication of the vaccine virus from day 21 onwards in both groups, coinciding with a progressive decrease of the BB ratio (Figure 1).
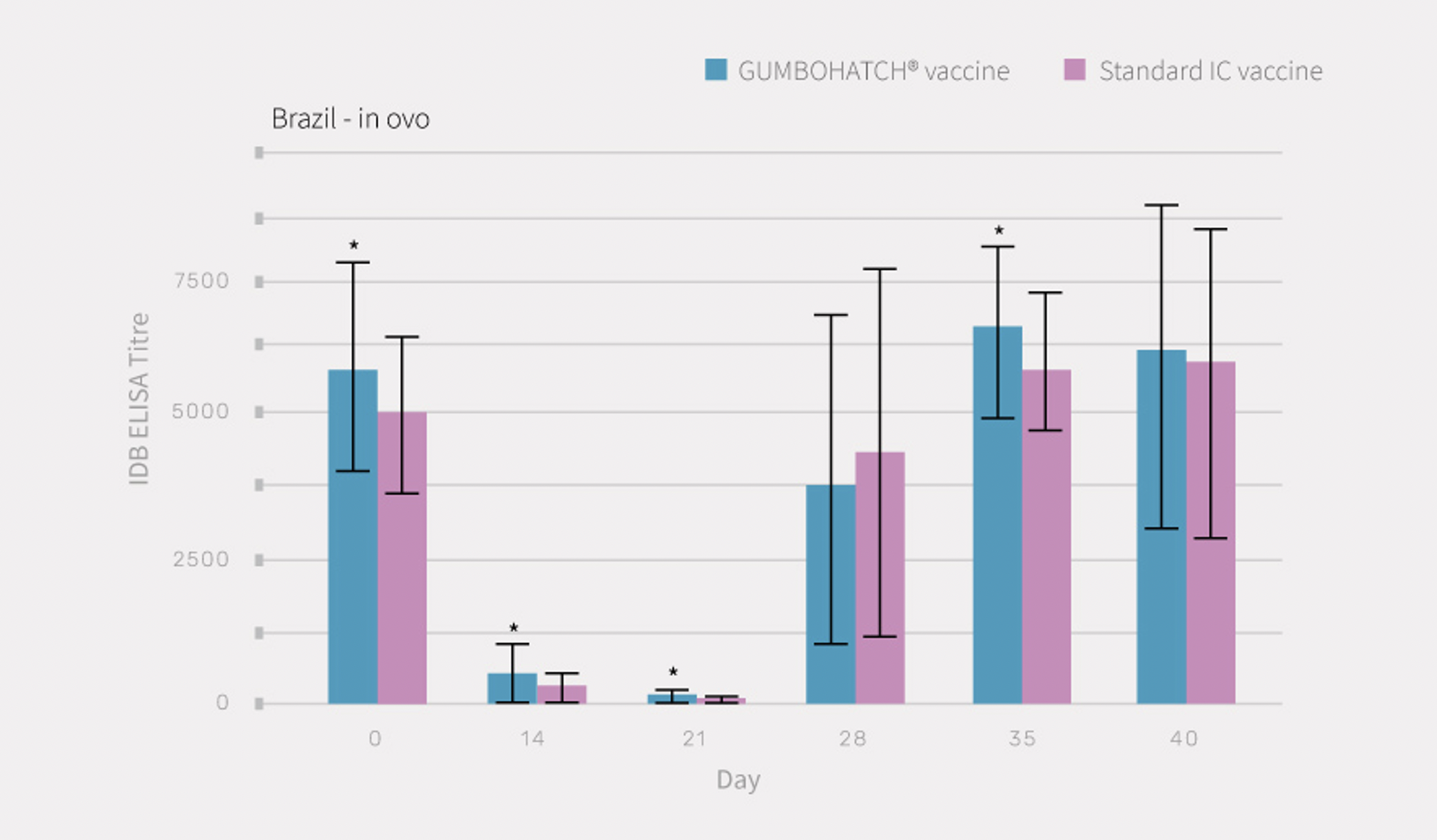
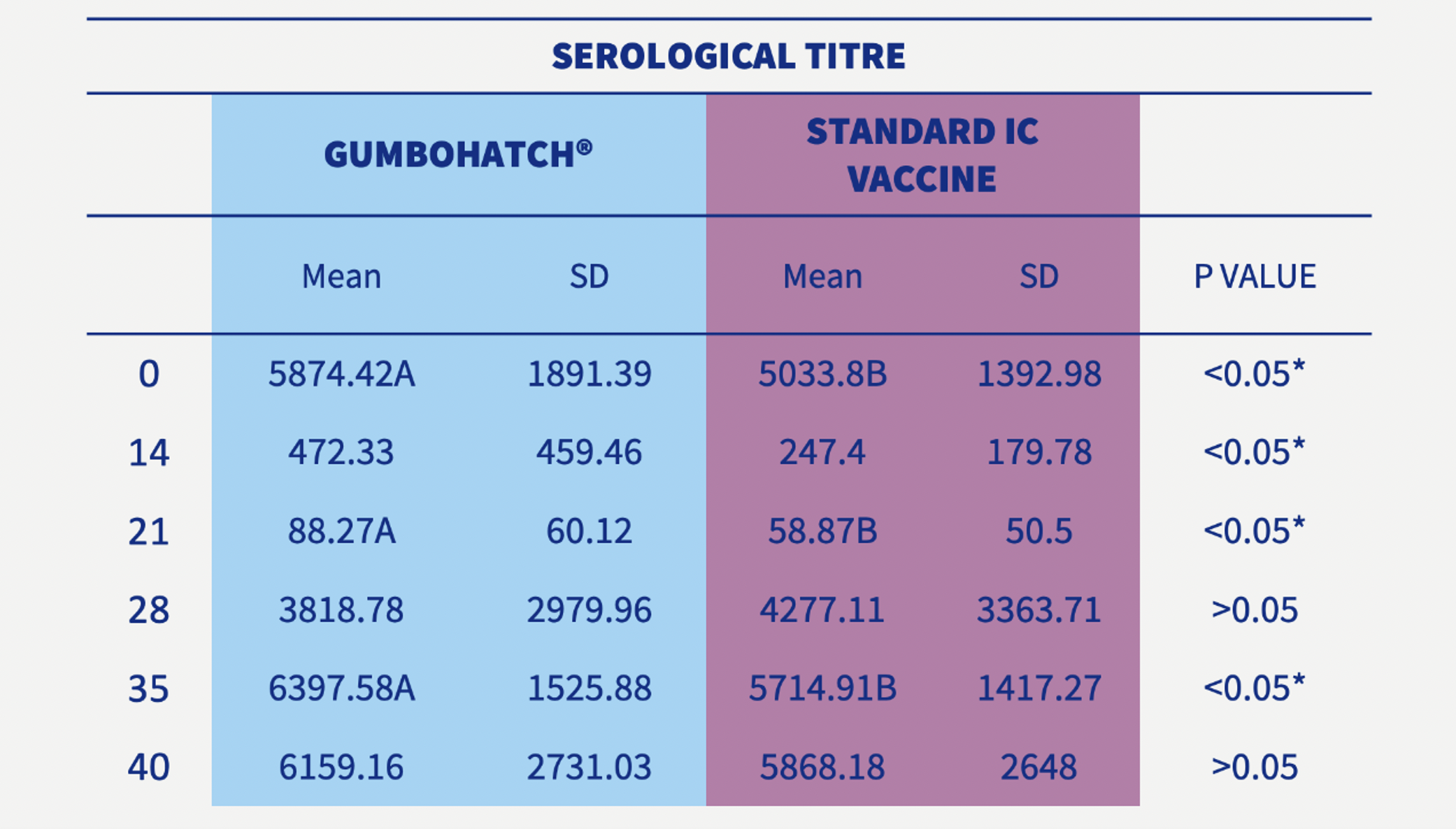
The development of antibody titres to the Gumboro virus after vaccination followed a similar pattern in both groups, with a progressive decrease in maternally-derived antibodies between days 0 and 21. However, in the case of the GUMBOHATCH® group, the maternally-derived antibodies were higher on each of the follow-up days (0, 14 and 21 days) compared with the standard formulated vaccine group.
The decrease in the maternally-derived antibodies was followed by a rapid increase in vaccine-induced antibodies from day 28 onwards up to the end of rearing.
Statistically significant differences (p<0.05) in vaccine-induced antibody titres were detected on day 35 in favour of the GUMBOHATCH® group (Figure 2), evidencing a faster humoral protection, even having always had higher levels of maternal antibodies.
So, which are the main improvements we can observe with GUMBOHATCH® in the field under Brazil conditions?
The results obtained in this study allow the conclusion to be drawn that vaccination with GUMBOHATCH® is safe and confers a faster humoral protection against Gumboro disease for the whole productive cycle of broiler chicks when administered via the in ovo route under field conditions.
The rapid humoral response observed with GUMBOHATCH®, compared to a standard formulated vaccine, even when higher maternal antibodies were present, may correspond to the new formulation and controls performed that prevent the neutralization of the vaccine virus when in contact with high levels of maternal antibodies.
References:
- Perozo et al. 2019. World Veterinarian Poultry Association Congress. 0262.
