Hatchery vaccination is becoming more and more popular thanks to the many advantages it provides in terms of vaccination precision and performance. In the case of vaccination against IBDV, it also offers the possibility of vaccinating in the presence of maternally derived antibodies whilst providing the reliability of individual injection.
The benefits of hatchery IBDV vaccination are clear, however 4 crucial points need to be observed if these benefits are to really be achieved:
Biosecurity and vaccine storage
Daily biosecurity procedures are considered to be the best management practice to reduce the possibility of introducing and spreading infectious diseases to hatcheries.
- Remember to follow external and internal biosecurity measures.
- Choose the most suitable vaccine for the prevention of IBDV and follow the special precautions for transportation and storage in accordance with the manufacturer’s instructions:
Check the temperature and appearance of the lyophilizate at the time of receipt of the vaccine.
> Ensure that the cold chain is not broken from the time the vaccine leaves the production facilities until it is applied. Discard a vaccine in which the cold chain has been broken.
> A maximum and minimum thermometer should be kept in the fridge to verify that temperatures are always within the acceptable ranges.
Vaccine reconstitution
Vaccine management, temperature, time elapsed after reconstitution, etc. are parameters to be evaluated on the day of vaccination to ensure the best results.
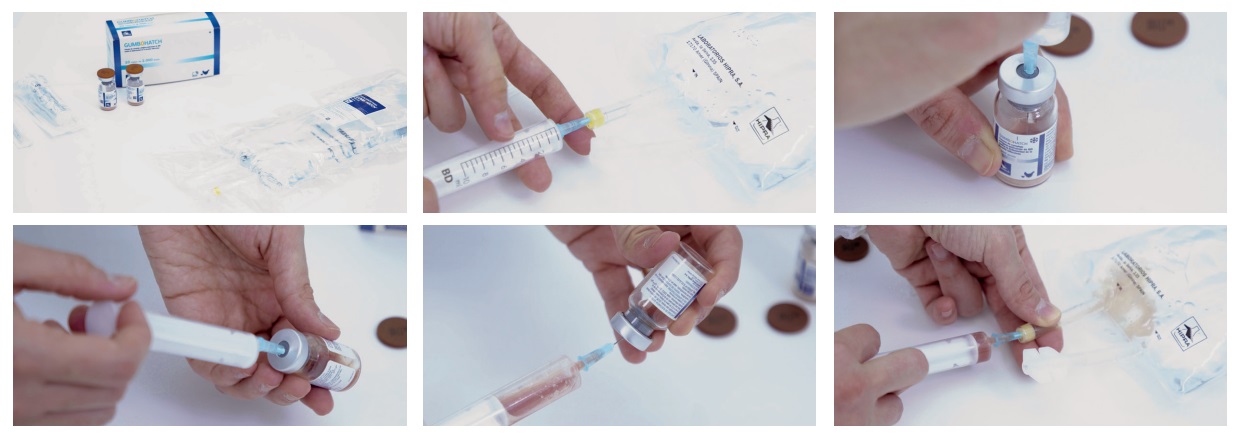
- Vaccines should be reconstituted in a clean room and operators should wash their hands before handling them.
- Clean the end caps (vaccine vial, solvent bag) with a disinfectant wipe before inserting the needle.
- Use single-use syringes and needles that are free of any disinfectants or chemical solutions.
- Calculate the required volume of vaccine to be used, taking into account the dosage that will be administered to the birds.
- Solvents require correct handling and use:
> Only the solvent specified by the manufacturer for the particular vaccine and presentation should be used.
> Solvents may also be sensitive to heat or freezing, thus transportation and storage measures should also be followed.
> Operators should be adequately trained to ensure that the solvent used is the correct one and that it is in good condition.
- Reconstitution: follow the manufacturer’s instructions, using sterile equipment free from any residues of chemical disinfectants.
- The vaccine should be inspected visually for any foreign particulate matter prior to administration. Reconstituted vaccines should be discarded after 1 hour of use. It is recommended that the bag should be labelled with the time of reconstitution.

Vaccine application
Correct quality management of the vaccines and correct application are crucial to ensure that the entire flock receives the correct dose.
- Hatchery IBDV vaccines, in general, are intended to be administered to embryonated eggs of 18 days’ development or to 1-day-old chicks.
- They usually allow successful early immunization of the chicks in the presence of MDA, and it is not necessary to calculate the appropriate time of vaccination.
- Administer the vaccine according to the manufacturer’s recommendations for the chosen route of administration (in-ovo or subcutaneous injection).
- Reconstituted solvent bags should be shaken every 15 min to avoid vaccine deposition during vaccination.
Vaccination audits and follow-up
Hatchery vaccination control audits should be carried out throughout the process to ascertain if there is room for improvement. In addition, the vaccination performance should also be analyzed to ensure that it was carried out correctly and that the animals are in good health and have a high productive performance.

The goal of IBDV vaccination follow-up is to monitor its effectiveness by evaluating different variables and critical control points:
- Equipment and materials, environment, vaccine storage and vaccination process.
- A dye should be mixed with the vaccine to allow visualization of the injection.
- Serological response, molecular diagnosis by PCR, macroscopic evaluation of the bursa of Fabricius, vaccination performance and zootechnical parameters (body weight, FCR, mortality, EPEF, uniformity…).
- The Global Hatchery Health Programme (GHHP) by HIPRA is a package of technical services focused on getting the most out of the incubation process by enhancing day-old chick health performance.
