The 4 key points for efficacious IBDV hatchery vaccination
4 March 2022
Hatchery vaccination is becoming more and more popular thanks to the many advantages it provides in terms of vaccination precision and performance. In the case of vaccination against IBDV, it also offers the possibility of vaccinating in the presence of maternally derived antibodies whilst providing the reliability of individual injection.
The benefits of hatchery IBDV vaccination are clear, however 4 crucial points need to be observed if these benefits are to really be achieved:
Daily biosecurity procedures are considered to be the best management practice to reduce the possibility of introducing and spreading infectious diseases to hatcheries.
Check the temperature and appearance of the lyophilizate at the time of receipt of the vaccine.
> Ensure that the cold chain is not broken from the time the vaccine leaves the production facilities until it is applied. Discard a vaccine in which the cold chain has been broken.
> A maximum and minimum thermometer should be kept in the fridge to verify that temperatures are always within the acceptable ranges.
Vaccine management, temperature, time elapsed after reconstitution, etc. are parameters to be evaluated on the day of vaccination to ensure the best results.
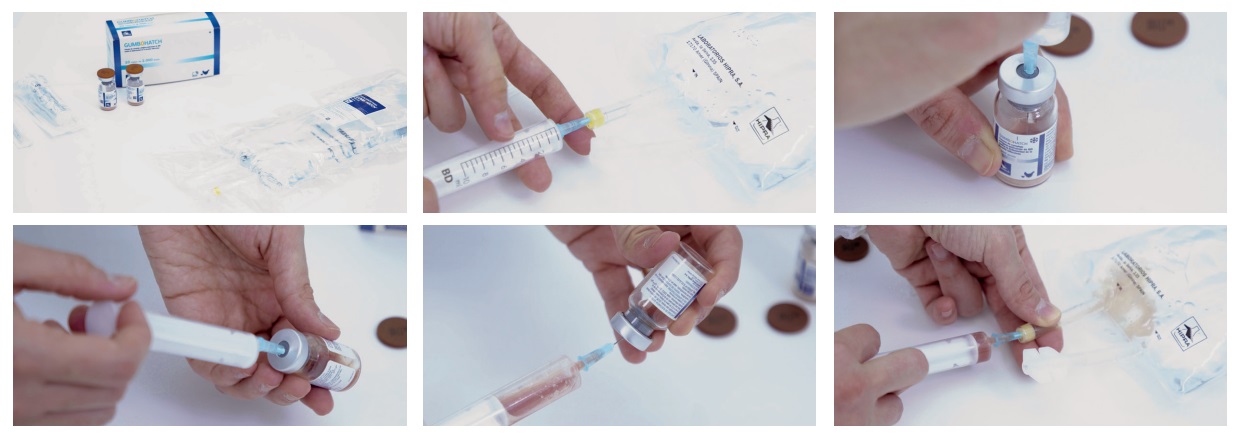
> Only the solvent specified by the manufacturer for the particular vaccine and presentation should be used.
> Solvents may also be sensitive to heat or freezing, thus transportation and storage measures should also be followed.
> Operators should be adequately trained to ensure that the solvent used is the correct one and that it is in good condition.

Correct quality management of the vaccines and correct application are crucial to ensure that the entire flock receives the correct dose.
Hatchery vaccination control audits should be carried out throughout the process to ascertain if there is room for improvement. In addition, the vaccination performance should also be analyzed to ensure that it was carried out correctly and that the animals are in good health and have a high productive performance.

The goal of IBDV vaccination follow-up is to monitor its effectiveness by evaluating different variables and critical control points:
Don't miss any updates
Controller: LABORATORIOS HIPRA, S.A.
Purposes: Managing the contractual and/or business relationship with HIPRA, including sending news, promotions and invitations to events sponsored by HIPRA.
Lawful basis: Performance of the contractual relationship and HIPRA’s legitimate Interest.
Recipients: Third parties to which HIPRA has entrusted cloud computing, security, auditing, mailing, technical and computer support services, as well as companies in its group.
Rights: Request access to and rectification or erasure of personal data and other rights as explained in the additional information. You can seeview the detailed additional information about data protection in our Privacy Policy.
For further information, please check our detailed information on Data Protection.