Reassortant very virulent IBDV in Europe. Is there a correlation between positivity on broiler farms and the type of vaccine currently used?
14 March 2024
Infectious bursal disease virus (IBDV) has a genome consisting of two segments of RNA (Müller et al. 2003). Segment A contains the VP2 protein, which is the main host protective antigen and is responsible for causing neutralizing antibody production (Dey et al. 2019). Segment B encodes protein VP1, which also contributes to the virulence and evolution of IBDV (Yang et al. 2020). The most common classification for IBDV is based solely on segment A, but, with the emergence of novel strains produced by mutation, recombination or reassortment events, it is increasingly difficult to define them using the traditional classification method.
In recent years, a reassortant IBDV strain has been continuously reported in many European countries (Tamas Mato et al. 2020).
Genetic analysis based on the VP2 gene classified these isolates as very virulent IBDV strains (vvIBDV, Genogroup 3), while the VP1 gene was more closely related to classical attenuated IBDV strains. Likewise, sequencing reports from bursas of Fabricius (BF) at HIPRA Diagnostic Services (HIPRA Diagnos) have repeatedly identified the sequence of IBD reassortant strains (named UK2019), linked to subclinical outbreaks and characterized by immunosuppressive signs, low performance and bursal atrophy (E. Marzo et al. 2023).
For this reason, a study was initiated on poultry farms in order to have an indication of the spread of this new virus in the Netherlands and to investigate whether there is a relationship between its appearance and the currently used vaccine strategy (P. Kühne et al. 2023).
FTA card samples were taken from 66 farms producing slow-growing broilers. The farms were randomly selected and included those with different vaccination strategies:
Seventy-six samples were sent to HIPRA Diagnos in Spain, all taken at least two weeks after the application of a Gumboro vaccine. The laboratory ran a RT PCR test, and in the case of a positive result, a nucleotide sequencing procedure was performed following the Sanger methodology. The sequences obtained were compared with both IBDV reference strains (Genbank) and field strains.
The percentage positivity for field virus UK2019 was analysed in all samples and by type of vaccine classification (Table 1):
These results indicate that vaccines with intermediate plus live viruses seem to be the best option for the control of these new strains, probably due to the important role that protection by competitive exclusion plays in this disease.
The positivity for field virus UK2019 by type of vaccine was 21.7% for the drinking water vaccines, 44.4% for the recombinant vaccines and 0% in immune complex vaccines (Fig. 1).
Furthermore, 33.3% of the recombinant vaccine samples showed positivity for a Winterfield 2512 strain (probably coming from another vaccine used in previous flocks). This result indicates that the protection observed from this vaccine type could be reduced to 22%.
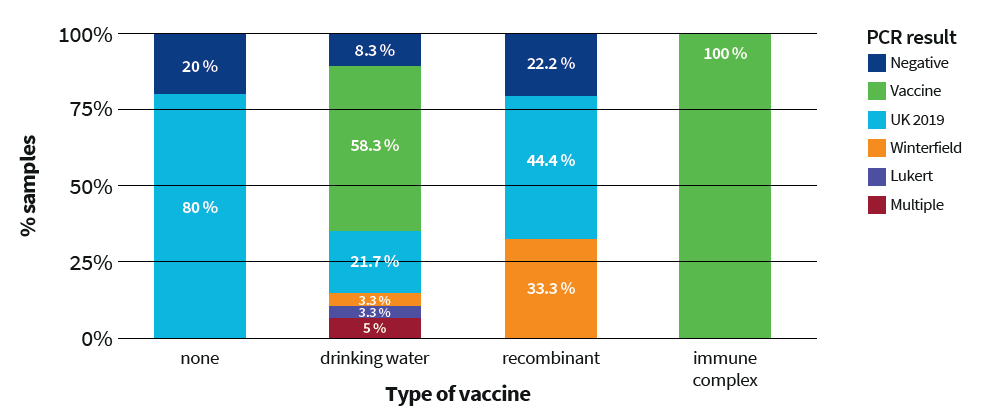
The results of this prevalence study confirmed the circulation of the new reassortant IBDV strain (UK2019) in the Netherlands and demonstrated a relationship between the positivity of the field strain and the type of vaccine used, indicating that intermediate plus vaccines, especially when in the form of immune complex vaccines, are the best option for controlling field circulation on farms producing slow-growing broilers.
Don't miss any updates
Controller: LABORATORIOS HIPRA, S.A.
Purposes: Managing the contractual and/or business relationship with HIPRA, including sending news, promotions and invitations to events sponsored by HIPRA.
Lawful basis: Performance of the contractual relationship and HIPRA’s legitimate Interest.
Recipients: Third parties to which HIPRA has entrusted cloud computing, security, auditing, mailing, technical and computer support services, as well as companies in its group.
Rights: Request access to and rectification or erasure of personal data and other rights as explained in the additional information. You can seeview the detailed additional information about data protection in our Privacy Policy.
For further information, please check our detailed information on Data Protection.