Immune complex vaccines against Gumboro: Is there room for improvement?
3 May 2021
Immune complex vaccines are nowadays market leaders in the fight against Gumboro disease. They’re the only type of vaccines that naturally adapt the onset of immunity to the protection that each individual chick needs. Their formulation is the key differential point, but how exactly are immune complex vaccines formulated? And more important, is there room for improvement?
Immune complex vaccines against Gumboro disease were developed in the late 1990s with the aim of having a biological product that could be administered in a hatchery providing protection and safety, regardless of the level of maternal antibodies in chicks.
The formulation of immune complex vaccines is based on the combination of a live attenuated vaccine strain with specific antibodies against Gumboro virus.
The coating of the virus with the antibodies is the key to guarantee the maintenance of the vaccine virus potency (as it confers the protection against the neutralisation by the maternal antibodies) and it also gives it its safety properties (by preventing the risk of a too early vaccine virus replication in the bursa that could lead to an effect of immunosupression1,3).
The main objective in the formulation of this type of vaccine is, therefore, to provide sufficient protection to the vaccine virus through its total coating with specific antibodies (Figure 1):
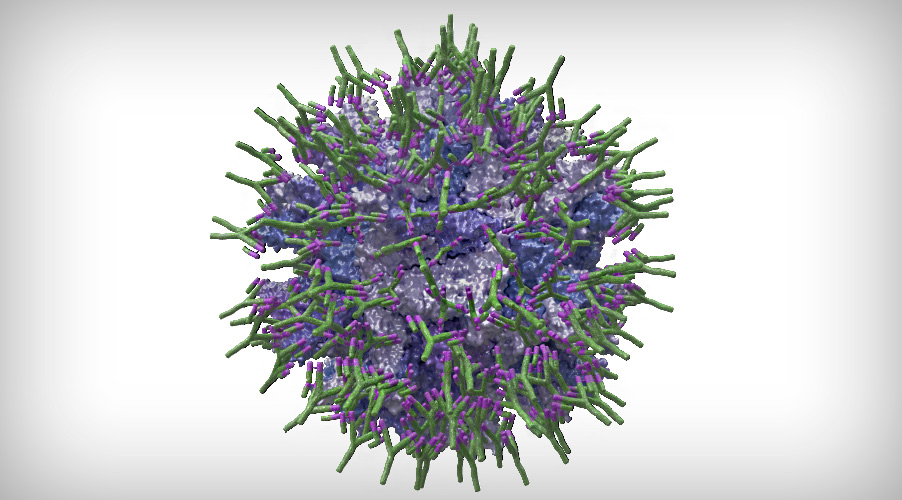 Figure 1. Simulation of a Gumboro virus completely coated with specific antibodies (IgY).
Figure 1. Simulation of a Gumboro virus completely coated with specific antibodies (IgY).
All immune complex vaccines against Gumboro disease are formulated by adding a specific proportion of antibodies, according to the initial titre of the vaccine virus culture (Figure 2).
This initial titre is usually determined in the titration substrate, such as embryonated chicken eggs (EID50: 50% infectious dose in egg or embryo) or cell lines (TCID50: dose that infects 50% of the culture tissue) in a similar way to the titration performed with conventional live vaccines.
Once the virus-specific antibody has been added, the mixture is normally subjected to a lyophilisation process, which can induce some loss of titre.
Some immune complex vaccines present this virus titre and amount of serum before lyophilisation in their technical specifications, without taking into account the loss of titre that may occur during the process or the correct coating of the virus.
In other cases, indirect titrations are carried out after lyophilisation by applying an ELISA test in birds free of specific pathogens (CID50: infectious dose in chicken at 50%), which can take into account the possible loss of titre, but, again, does not guarantee that all virus particles are coated with specific antibodies (the main objective of the formulation of immune complex vaccines).
 Figure 2. Basis of the formulation of immune complex vaccines against Gumboro virus. Although the main objective of the process is to ensure the potency and safety of the vaccine with a fully coated virus, the final controls implemented do not guarantee this.
Figure 2. Basis of the formulation of immune complex vaccines against Gumboro virus. Although the main objective of the process is to ensure the potency and safety of the vaccine with a fully coated virus, the final controls implemented do not guarantee this.
A fundamental point to introduce in the formulation of immune complex vaccines is the control of the correct coating of the viral particles.
Correct coating of the virus is the only way to ensure that the immune complex vaccine will obtain homogeneous results in the field, since it is this what will prevent the possible loss of vaccine titre when the virus is exposed to high levels of maternal antibodies.
In addition, it is also the only way to avoid safety risks (too early replication of the vaccine virus in the bursa) when the maternal antibodies are low.
Two new controls for a new generation immune complex vaccine (GUMBOHATCH®) have recently been developed to achieve this goal:
However, new tests and additional formulations improvements have also been introduced to make the production process for immune complex vaccines better and more consistent:
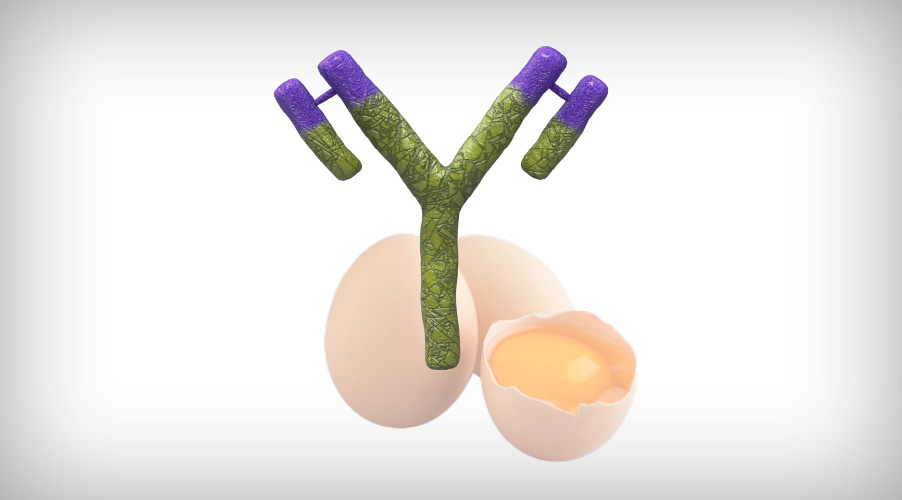 Figure 3: A new immune complex vaccine has been developed that includes IgY of egg origin.
Figure 3: A new immune complex vaccine has been developed that includes IgY of egg origin.
All of these new improvements have served to produce a new generation immune complex vaccine (GUMBOHATCH®) that, by guaranteeing the complete coating of the vaccine virus, ensures the maintenance of the maximum potency of the vaccine, consistent results in the field and the possibility to be administered at any levels of initial maternal antibodies, being the first immune complex in Europe to achieve that goal!.
References:
Don't miss any updates
Controller: LABORATORIOS HIPRA, S.A.
Purposes: Managing the contractual and/or business relationship with HIPRA, including sending news, promotions and invitations to events sponsored by HIPRA.
Lawful basis: Performance of the contractual relationship and HIPRA’s legitimate Interest.
Recipients: Third parties to which HIPRA has entrusted cloud computing, security, auditing, mailing, technical and computer support services, as well as companies in its group.
Rights: Request access to and rectification or erasure of personal data and other rights as explained in the additional information. You can seeview the detailed additional information about data protection in our Privacy Policy.
For further information, please check our detailed information on Data Protection.